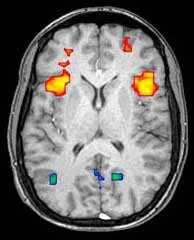
What is BOLD fMRI?
Since the development of blood oxygenation level dependent (BOLD) contrast scanning methods in the early 1990s, functional MRI (fMRI) has grown to become a pillar of neurocognitive research. It has also entered into widespread clinical use for mapping eloquent brain cortex in preparation for neurosurgery for brain tumors or epilepsy. Nevertheless, challenges in calibrating and quantifying BOLD fMRI signal remain an obstacle to applying this technique for the diagnosis of neuropsychiatric diseases in individual patients.
Molecular conformational changes to hemoglobin form the basis of fMRI. Oxygenated hemoglobin is diamagnetic whereas deoxygenated hemoglobin is paramagnetic. Therefore, deoxyhemoglobin causes local dephasing of spinning proton dipoles and loss of T2* MRI signal. As a result of cerebrovascular autoregulation, brain regions that are metabolically active as a result of a stimulus or cognitive effort receive relatively more blood than non-active regions. The complex biochemical signaling system that permits this redirection of blood flow to metabolically active brain remains a matter of ongoing investigation but likely involves chemical mediators such as nitrous oxide and glutamate as well as the participation of astrocytes.
Regardless the exact mechanism, active neurons drive a regional increase in cerebral blood flow that overcompensates for the increased local oxygen demand. Therefore, a local increase in the oxyhemogloblin to deoxyhemoglobin ratio in metabolically active brain results in increase in T2* signal as detected by MRI. These T2* signal changes, which vary from about 2% on a 1.5 Tesla MRI scanner about 12% on a 7 Tesla MRI scanner, can be detected using a T2* sensitive scanning technique such an echo planar-gradient echo (EPI/GRE) pulse sequence.
The most common means of acquiring an fMRI scan involves scanning the brain repeatedly with an EPI-GRE pulse sequence. Each individual scan through the brain takes 333-3000 milliseconds depending on the scanner model and pulse sequence employed.
While the brain is scanned over and over again during a period of 4 to 10 minutes, the individual being scanned is presented with cognitive stimuli or prompts to perform specific motor tasks such as moving a hand or leg. The stimuli most commonly consist of visual images presented with commercially available MRI compatible video goggles, digital projectors, or LCD screens. Alternatively, auditory stimuli can be provided with specialized headphones or ear buds. Periods of stimulus presentation, which are precisely synchronized with scanning, can be delivered in a series of blocks or epochs occurring at fixed time intervals (e.g. every 20 seconds). Such a predictable presentation scheme is referred to as a “block paradigm”. Alternatively, the stimuli can be presented at random or pseudorandom time points during scanning with this being known as an “event- related paradigm.” Block paradigms provide greater statistical power in identifying active brain whereas event related paradigms provide a more naturalistic stimulus presentation.
Once images are acquired, processing and analysis can be accomplished using a variety of software packages. Some of these software systems are freely distributed by the research community whereas other regulatory approved software products designed for clinical application are available from commercial vendors. In general, these fMRI analysis software programs perform two functions. First, the raw fMRI images are preprocessed in an effort to correct for such factors as patient head motion, slice timing differences, and various sources of image noise or artifact. In the research setting, preprocessing may include alignment of the images to a common anatomic space or atlas. The second major analysis task is derivation of the statistical maps which form the fMRI “images”. This is accomplished by voxel-wise statistical comparison of T2* signal in the brain images acquired during stimuli presentation with those obtained during absence of the stimuli. A variety of statistical methodologies have been advocated including the general linear model, correlation analysis, or simple voxel wise t-tests. The final output is a map that shows voxels in which stimulus related brain activation is considered to be statistically significant based on a specified threshold such as a p-value, t-value or z-score with the chosen measure depending on the specific statistical model employed. For neurocognitive research applications, it is common to employ a uniform, fixed statistical threshold such as p=0.001 across all subjects as well as some form of correction for multiple comparisons. However, given inter-individual variability in cerebrovascular responsiveness, the statistical threshold generally requires customization for clinical work in single patients.
For neuropsychiatric disease research, a common study approach is to create group summary statistical maps that represent composites of the subject and control groups. These composite group maps are then statistically compared to identify brain sites of activation difference between the individuals affected by some disease condition with those of controls. While such studies provide invaluable information on the pathophysiology of neuropsychiatric disease, in most cases, their results cannot be applied to the diagnosis of the researched disease in an individual patient because of inability to appropriately calibrate for variable physiology in the BOLD response among individuals as well as other acquisition related factors inherent to the MRI scanner, pulse sequence, and RF coil.
Nevertheless, for individual patients, BOLD fMRI is highly valuable for mapping cerebral motor, language, and visual cortex in anticipation of brain surgery. Such maps of eloquent cortex can help to define surgical risk, aid with operative planning, and expedite intracranial cortical stimulation mapping at the time of surgery. fMRI when combined with DTI has been shown to reduce the rate of postoperative neurologic deficits. Often, fMRI exams are combined with diffusion tensor imaging in preoperative surgical mapping as a means of depicting both cortex and white matter tracts at risk. Many neurosurgeons elect to load fMRI statistical maps into neurosurgical navigation systems. However, this needs to done with the understanding that the fMRI images are statistical activation maps displayed at an arbitrary threshold rather than a direct representation of anatomic structures.
Another clinical application of fMRI is mapping sites of cerebrovascular reactivity in patients with vascular diseases such as moyamoya or atherosclerosis. By inducing hypercapnia, either through timed breath-holding or through use of an MR compatible gas delivery device, a vasodilatory stimulus can be delivered to the entire brain simultaneously. Brain regions that fail to demonstrate BOLD signal rise in response to hypercapnia correlate with areas lacking cerebrovascular reserve or regions that are located in zones of magnetic susceptibility that obscure the BOLD signal.
A number of new horizons are opening in the arena of fMRI that promise to increase the value of this technique in the future. Ultrahigh field MRI scanners such as new 7 Tesla models allow for higher spatial resolution of the BOLD response as well as activation maps of higher statistical significance. New pulse sequences such as multiband-multiecho promise to improve the quality of raw fMRI data including reduction in undesirable venous signal contribution. Efforts at acquisition standardization and signal calibration may allow for a more quantitative analysis of BOLD signal in individual patients and may eventually enable fMRI to diagnose neuropsychiatric diseases. Such developments predict the ongoing importance of fMRI, both to neuroscientists and clinical neuroradiologists.
Black, D.F., Vachha, B., Mian, A., Faro, S.H., Maheshwari, M., Sair, H.I., Petrella, J.R., Pillai, J.J. and Welker, K., 2017. American Society of Functional Neuroradiology–Recommended fMRI Paradigm Algorithms for Presurgical Language Assessment. American Journal of Neuroradiology, 38(10), pp.E65-E73.
Benjamin, C.F., Walshaw, P.D., Hale, K., Gaillard, W.D., Baxter, L.C., Berl, M.M., Polczynska, M., Noble, S., Alkawadri, R., Hirsch, L.J. and Constable, R.T., 2017. Presurgical language fMRI: mapping of six critical regions. Human brain mapping, 38(8), pp.4239-4255.
Peacock, J., D. Black, D. DeLone, and K. Welker. "Use of a Simple Breath-Holding Task for Cerebrovascular Reactivity Scans in Clinical Functional MR Imaging." Neurographics 6, no. 4 (2016): 213-218.
Nadkarni, T.N., Andreoli, M.J., Nair, V.A., Yin, P., Young, B.M., Kundu, B., Pankratz, J., Radtke, A., Holdsworth, R., Kuo, J.S. and Field, A.S., 2015. Usage of fMRI for pre-surgical planning in brain tumor and vascular lesion patients: task and statistical threshold effects on language lateralization. NeuroImage: Clinical, 7, pp.415-423.
Pillai, J.J. and Mikulis, D.J., 2015. Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. American Journal of Neuroradiology, 36(1), pp.7-13.
Lazar, N., 2008. The statistical analysis of functional MRI data. Springer Science & Business Media.
Voyvodic, J.T., 2006. Activation mapping as a percentage of local excitation: fMRI stability within scans, between scans and across field strengths. Magnetic resonance imaging, 24(9), pp.1249-1261.
Petrella JR, Shah LM, Harris KM, Friedman AH, George TM, Sampson JH, Pekala JS, Voyvodic JT. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006 Sep;240(3):793-802. Epub 2006 Jul 20.
Gaillard, W.D., Balsamo, L., Xu, B., McKinney, C., Papero, P.H., Weinstein, S., Conry, J., Pearl, P.L., Sachs, B., Sato, S. and Vezina, L.G., 2004. fMRI language task panel improves determination of language dominance. Neurology, 63(8), pp.1403-1408.
Medina LS, Bernal B, Dunoyer C, et al. Seizure disorders: functional MR imaging for diagnostic evaluation and surgical treatment-prospective study. Radiology 2005;236:247-253.
Medina LS, Aguirre E. Bernal B, Altman NR. Functional MR imaging versus Wada test for evaluation of language lateralization: cost analysis. Radiology 2004;230:49-54.
Hirsch J, Ruge MI, Kim KH, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery 2000;47:711-721.
Tomczak RJ, Wunderlich AP, Wang Y, et al. fMRI for preoperative neurosurgical mapping of motor cortex and language in a clinical setting. J Comput Assist Tomogr 2000;24:927-934.
Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional MRI. J Neurosci. 1997;17: 353–362.
Bandettini PA, Wong EC, Hinks RS, Rikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992; 25:390–397.
Ogawa S, Lee T-M, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872.
Thulborn KR, Waterton JC, Mattews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochem Biophys Acta. 1982;714:265–270.